Home
Goals
Partners
Publications
Results
Gallery
Open position
Contact
Internal
Workshop 2006
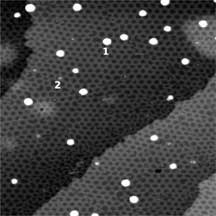
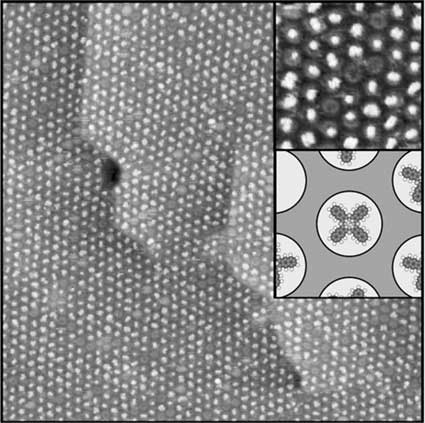
The boron nitride nanomesh acts as a scaffold for metal clusters and molecules
The nanomesh pore size of 2 nm makes it as a promising template for a periodic arrangement
of molecules or metallic clusters, what is nowadays an important challenge in
nanotechnology.
And it works out effectively!
The nanomesh is stable under different environments like normal air, water and even acids. In addition, it doesn't decompose up to 1275K under vacuum. This exceptional stability opens a wide range of potential applications of the nanomesh as a scaffold. Here we present two of them.
Such systems with wide spacing between individual molecules/clusters and negligible intermolecular interactions might be interesting for applications such as molecular electronics and memory elements, in photochemistry or in optical devices. The remarkable stability of a single h-BN monolayer on Rh and Ru paves the way for applications of this material as a template that is not based on expensive ultrahigh vacuum deposition techniques, but on deposition from aqueous solutions, what is much cheaper and therefore commercially very interesting. |
|||||
© 2007 University of Zurich
|
Webmaster
|
| Last update: 25.03.2008 by C. Galli Marxer
|